Ideal Gas Equation
Ideal Gas Equation: Overview
This Topic covers sub-topics such as Ideal Gas Equation, Ideal Gas, Universal Gas Constant, Combined Gas law, Partial Pressure in Terms of Mole Fraction, Density and Molar Mass Relation, Derivation of Dalton's Law and, Calculation of Universal Gas Constant
Important Questions on Ideal Gas Equation
A gaseous mixture was prepared by taking equal mole of If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen in the mixture is:
If 500ml of gas A at 400 torrs and 666.6 ml of B at 600 torrs are placed in a 3 liter flask, the pressure of the system will be
If 500ml of gas A at 400 torr and 666.6 ml of B at 600 torr are placed in a 3 litre flask, the pressure of the system will be
Cyclopropane and oxygen at partial pressures torr and torr respectively are mixed in a gas cylinder. What is the ratio of the number of moles of cyclopropane to the number of moles of oxygen (Assume no reaction).
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
If are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
Under what conditions can ideal gases be liquefied? Give reasons to support your answer.
The mass of of a triatomic elemental gas at and is . Calculate the weight of one atom.
The vapour density of hydrogen peroxide is equal to molecular weight of .
Equal masses of the three gases, ammonia, nitrogen dioxide and methane are taken in three cylinders having a volume ratio of at a certain temperature. Draw a comparison of the pressures exerted by those gases.
The volume of a given mass of a gas at is . To what temperature should it will be heated at the same pressure so that it will occupy a volume of ?
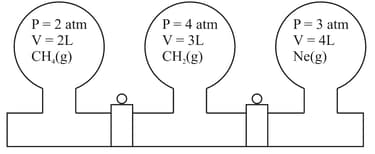
Three bulbs are filled with CH4, CO2 and Ne as shown in the picture. The bulbs are connected through pipes of zero volume. When the stopcocks are opened and the temperature is kept constant throughout, the pressure of the system is found to be______ atm. (Nearest integer).
A fixed mass of an ideal gas is maintained at constant volume. The pressure of the gas at the triple point of water is . What is the thermodynamic temperature of the gas when its pressure is ?
A mixture if hydrogen and oxygeb hs volume , temperature , pressure and mass .Calculate the masses of hydrogen and oxygen in the mixture.
Calculate the number of molecules in one litre of an ideal gas at temperature and pressure.
Three flasks of identical volume are filled separately by (a) gram of (b) (b) gram of (c) gram of
They are immersed in a tank of water so that all of them attain same temperature. The pressures are related as
The density of oxygen is at STP. The density of oxygen at and torr is:
Find out the volume of of at a temperature of and of pressure.
At temperature in a closed vessel and of gases are filled. Find the partial pressure and total pressure of a mixture.
The composition by volume of are in by proportion the total pressure is bar find the partial pressure of each gas
